Detection of arbuscular mycorrhizal fungi in the litter of different tree species: review of three clarification techniques.
DOI:
https://doi.org/10.31055/1851.2372.v58.n2.37810Keywords:
arbuscular mycorrhizal fungi, clarification techniques, fungal structures, histochemistry, leaf litterAbstract
Background and aims: Arbuscular mycorrhizal fungi (AMF) are obligate symbionts with plant roots, and they colonize the surrounding soil and leaf litter. A unique clarification technique has been used for studying AMF in decomposing leaves, but it is not optimal for all types. This study aimed to evaluate and adjust three clearing techniques on leaf litter of diverse plant species for the detection of AMF structures.
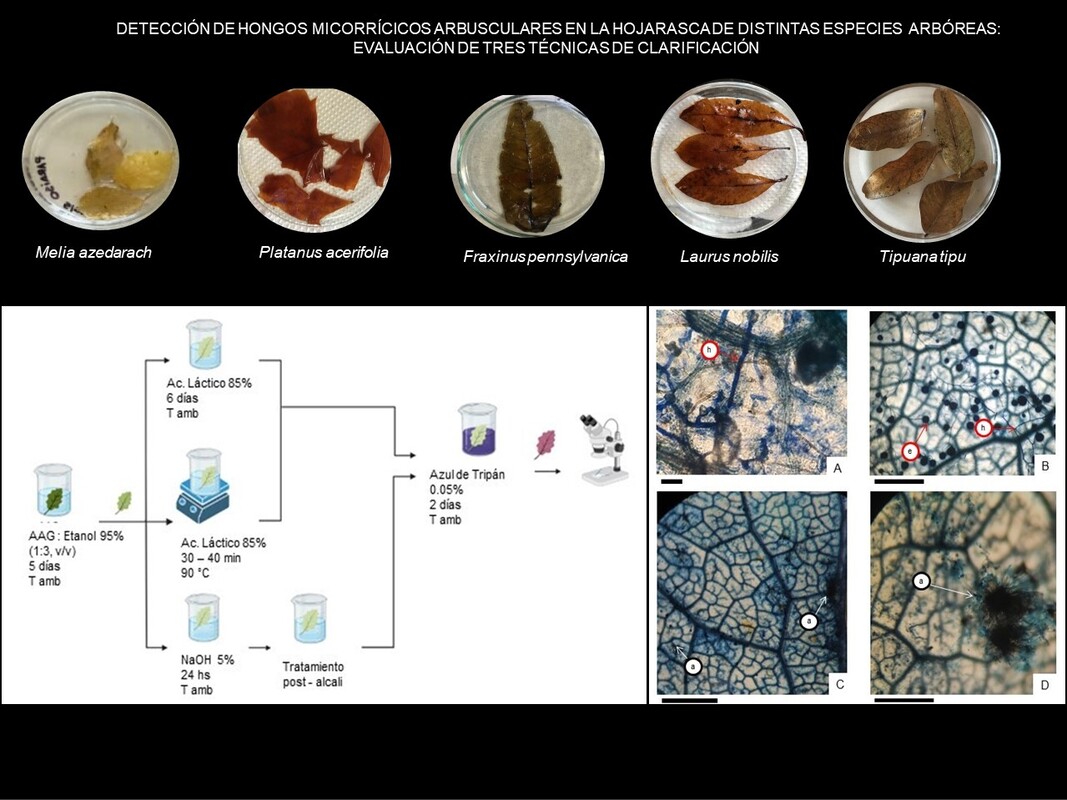
M&M: We collected leaf litter of Quercus robur, Tipuana tipu, Ulmus minor, Fraxinus pennsylvanica, Platanus acerifolia, Laurus nobilis, Populus alba and Melia azedarach from soil surface of two parks in Buenos Aires (Argentina). Also, we inoculated leaf litter of F. pennsylvanica with Rhizoglomus intraradices in a pot culture under semi- controlled conditions. Three clarification techniques were tested: the “5-5-5 staining technique” by Arambarri, the root clarification technique by Phillips & Hayman, and Peterson et al. The time and temperature to reagent exposure were adjusted according to each decomposing leaf.
Results: The clarification technique of Peterson et al. was the most appropriate for all the tested leaf litters. An effective clarification, conservation of leaf structure and visualization of extraradical spores and mycelia of AMF was achieved.
Conclusions: The selection of the appropriate technique greatly depends on the quality and composition of leaf litter. By the optimization of Peterson et al. Technique, we were able to detect structures of AMF on decomposing leaves of F. pennsylvanica and T. tipu.
References
ARAMBARRI, A.M. 2018. La "técnica de clarificación 5-5-5", un método natural para el tratamiento de material vegetal. Bol. Soc. Argent. Bot. 53: 579-586. http://dx.doi.org/10.31055/1851.2372.v53.n4.21980.
ARISTIZABAL, C., E.L. RIVERA & D.P. JANOS. 2004. Arbuscular mycorrhizal fungi colonize decomposing leaves of Myrica parvifolia, M. pubescens and Paepalanthus sp. Mycorrhiza 14: 221-228. https://doi.org/10.1007/s00572-003-0259-0
BUNN, R.A., D.T. SIMPSON & L.S. BULLINGTON. 2019. Revisiting the 'direct mineral cycling' hypothesis: arbuscular mycorrhizal fungi colonize leaf litter, but why? ISME J. 13: 1891-1898. https://doi.org/10.1038/s41396-019-0403-2
CAO, T., Y. CHENG, Y. FANG, X. KONG, J. YANG, H. ALHARBI, Y. KUZYAKOV & X. TIAN. 2022. Synergy of saprotrophs with mycorrhiza for litter decomposition and hotspot formation depends on nutrient availability in the rhizosphere. Geoderma 410:115662. http://dx.doi.org/10.1016/j.geoderma.2021.115662
CHOWDHURY, S., M. LANGE, A.A. MALIK, T. GOODALL, H. JIANBEI, R.I. GRIFFITHS & G. GLEIXNER. 2022. Plants with arbuscular mycorrhizal fungi efficiently acquire Nitrogen from substrate additions by shaping the decomposer community composition and their net plant carbon demand. Plant Soil 475: 473-490. https://doi.org/10.1007/s11104-022-05380-x
CHURCHLAND, C. & S. GRAYSTON. 2014. Specificity of plant-microbe interactions in the tree mycorrhizosphere biome and consequences for soil C cycling. Front. Microbiol. 5: 261. https://doi.org/10.3389/fmicb.2014.00261
DÍAZ ARIZA, L.A., E.L. RIVERA & N. SÁNCHEZ. 2021. Occurrence of arbuscular mycorrhizal fungi in leaf litter and roots of shaded coffee plantations under organic and conventional management. Rev. Bras. Cienc. Solo 45: e0200110. https://doi.org/10.36783/18069657rbcs20200110
GARDNER, R.O. 1975. An overview of botanical clearing techniques. Stain. Technol. 50: 99-105. https://doi.org/10.3109/10520297509117042
GOUGOULIAS, C., J.M. CLARK & L.J. SHAW. 2014. The role of soil microbes in the global carbon cycle: tracking the below-ground microbial processing of plant-derived carbon for manipulating carbon dynamics in agricultural systems. J. Sci. Food Agric. 94: 2362-71. https://doi.org/10.1002/jsfa.6577
KOBAYASHI, Y., T. MAEDA, K. YAMAGUCHI, H. KAMEOKA, S. TANKA, T. EZAWA, S. SHIGENOBU M. & KAWAGUCHI. 2018. The genome of Rhizophagus clarus HR1 reveals a common genetic basis for auxotrophy among arbuscular mycorrhizal fungi. BMC Genomics 19: 465. https://doi.org/10.1186/s12864-018-4853-0
LINLIN SHI, L., G.G.O. DOSSA, E. PAUDEL, H. ZANG, J. XU & RD. HARRISON. 2019. Changes in fungal communities across a forest disturbance gradient. Appl. Environ. Microbiol. 85: e00080-19. https://doi.org/10.1128/AEM.00080-19
MEI, L., P. ZHANG, G. CUI, X. YANG, T. ZHANG & J. GUO. 2022. Arbuscular mycorrhizal fungi promote litter decomposition and alleviate nutrient limitations of soil microbes under warming and nitrogen application. App. Soil. Ecol. 171: 104318. https://doi.org/10.1016/j.apsoil.2021.104318
PETERSON, R.L., C.A. PETERSON & L.H. MELVILLE. 2008. Teaching plant anatomy through creative laboratory exercises. NRC ResPress. Ottawa, Ontario, Canada.
PHILLIPS, J.M. & D.S. HAYMAN. 1970. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Brit. Mycol. Soc. 55: 158-161. https://doi.org/10.1016/S0007-1536(70)80110-3
PLENCHETTE, C., & C. MOREL. 1996. External phosphorus requirement of mycorrhizal and non-mycorrhizal barley and soybean plants. Biol. Fertil. Soils 21: 303-308. https://doi.org/10.1007/BF00334907
SMITH, S.E., & D. READ. 2008. Mycorrhizal Symbiosis. 3rd ed. Elsevier Ltd, London.
TISSERANT, E., A. KHOLER, P. DOZOLME-SEDDAS, R. BALLESTRINI… & F. MARTIN. 2011. The transcriptome of the arbuscular mycorrhizal fungus Glomus intraradices (DAOM 197198) reveals functional tradeoffs in an obligate symbiont. New Phytol. 193: 755-769. https://doi.org/10.1111/j.1469-8137.2011.03948.x
VALDÉS, F.E., C. ABARCA, R.P. COLOMBO & V.A. SILVANI. 2020. Capítulo 1: Introducción y generalidades. En: SAPARRAT M.C.N., M.F. RUSCITTI & M.C. ARANGO (eds.). Micorrizas arbusculares: Biología y aplicaciones en el sector agroforestal. Buenos Aires: Editorial Edulp.
Downloads
Published
Issue
Section
License
Copyright (c) 2023 Sofía Crescio, Alicia Margarita Godeas, Vanesa Silvani

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Provides immediate and free OPEN ACCESS to its content under the principle of making research freely available to the public, which fosters a greater exchange of global knowledge, allowing authors to maintain their copyright without restrictions.
Material published in Bol. Soc. Argent. Bot. is distributed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International license.




